Abstract
Introduction
Diffuse large B cell lymphoma (DLBCL) is an aggressive hematological malignancy where patients rapidly progress on standard therapies. In relapsed/refractory (r/r) DLBCL, there is an urgent need for safe and effective therapies, especially being feasible to combine with other treatment regimens. The gemcitabine (GEM) oxaliplatin (OX) chemotherapy regimen is a standard treatment option with modest side effects providing an opportunity for potential combination with novel agents.
The PI3Kδ selective inhibitor linperlisib is a structurally distinct oral agent previously demonstrated to be clinically efficacious with a favorable safety profile in follicular lymphoma and peripheral T cell lymphoma trials.
Methods
This Phase1b/2 trial (NCT04500561) initiated in December 2019. As of July 22, 2021, there were 39 r/r DLBCL patients (pts) enrolled at 6 clinical sites in China. All DLBCL subtypes were represented, and pts had a median of 2 prior systemic therapies, including 1 pt with prior stem cell transplant. Pts were dosed in a 21-day cycle with linperlisib 80 mg QD orally combined with 6 cycles of GEMOX (Gemcitabine 1000 mg/m 2, IV on day 1, Q3W, and Oxaliplatin 1000 mg/m 2 IV on day2, Q3W], followed by linperlisib alone until disease progression, intolerable toxicity, or withdrawal. Safety was evaluated according to NCI-CTCAE v5.0. Efficacy evaluation was conducted every 2 cycles by IWG 2007 criteria.
Results
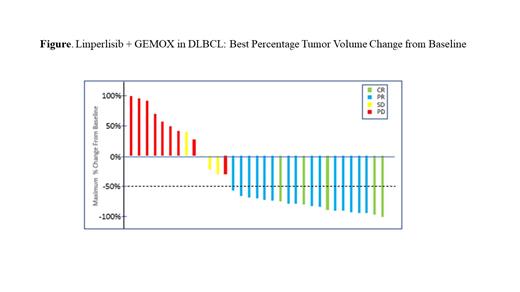
In this Phase 1b study of linperlisib + GEMOX in r/r DLBCL, as of July 22, 2021, there were 35 evaluable pts. Two pts discontinued from study due to SAEs occurred at C1D16 and C1D15, and 2pts discontinued from the study due to withdrawing consent at C1D8 and C2D7, there were no post baseline imaging assessment for these patients.The r/r DLBCL pts were BCL-6 (+) (80%, 28 pts), BCL-6 (-) (11.4%, 4 pts), or unknown (8.6%, 3 pts); C-MYC (+) (80%, 28 pts), C-MYC (-) (2.8%, 1 pt), or unknown (17.1%, 6 pts), indicative of aggressive lymphomas. Most pts (74%,29pts) had received R-CHOP as a first line treatment, with later lines of R-EPOCH, R-ICE, lenolinamide, CAR-T, PD-1, and other regimens, with a median of 2 lines of prior systemic therapy. The overall response rate was 60% (21/35 pts) with 5 pts achieving a Complete Response (CR, 14%) and 16 pts with Partial Response (PR, 46%)(Figure 1). In addition, 4 pts had Stable Disease (SD, 11 %), with a 71% Disease Control Rate (DCR). The median time to response was 36 days. As of the data cutoff date, the median Progression Free Survival was 193 days and the median Duration of Response was not reached, and 17 evaluable pts (48.6%) remained on study. Ten pts received linperlisib monotherapy for at least 1 cycle after completing 6 cycles of linperlisib + GEMOX combination therapy, and 2 pts were continuing linperlisib monotherapy treatment for >9 months after enrolling.
Linperlisib in combination with GEMOX was well-tolerated. All 39 pts experienced at least 1 Treatment Related Adverse Event (TRAE), of which the majority were Grade 1 or 2 (83.9%). The most common TRAEs (>10% all grades) were neutropenia (72%), leukopenia (62%),thrombocytopenia (56%), anaemia (51%), lymphopenia (15%), ALT increased (41%), ASP increased(54%), hyperamylasemia (23%), hypokalaemia (21%), nausea(31%), vomiting(23%), diarrhea(33%), and anaesthesia(13%).The most frequent ≥Grade 3 TRAE (>5%) were neutropenia (46.2%), thrombocytopenia (35.9%), leukopenia (25.6%), lymphopenia (7.7%). SAEs were reported in 14 pts, including 3 thrombocytopenia, 2 diarrhea, and one case of leukopenia, neutropenia, febrile neutropenia, fever, death NOS, cachexia, pleural effusion, cancer pain, hypotension, vomiting, laryngopharyngitis, pneumonitis, lung infection or skin infection. One pt had dose reduction to 60mg/day due to AE, 2 pts discontinued treatment due to a TRAE. The AEs observed were consistent with that in previous linperlisib monotherapy trials in FL, PTCL and lymphomas.
Conclusions
The combination of linperlisib and GEMOX treatment was well tolerated with a manageable AE profile, and led to a promising ORR in a difficult-to-treat r/r DLBCL. The preliminary data suggest that linperlisib + GEMOX is a safe and active combination. A Phase2 study of linperlisib in combination with GEMOX in r/r DLBCL is currently ongoing. Further, multiple combination treatments are planned as important avenues for future development of linperlisib.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal